Shop
(Box) Status™ COVID-19/Flu A&B Combo Rapid Test Kit CLIA Waived, 25/Bx
SKU:
BB-CF01
(Box) Status Flu A & B Rapid Test Kit CLIA Waived, 25/Bx
SKU:
BB-FL02
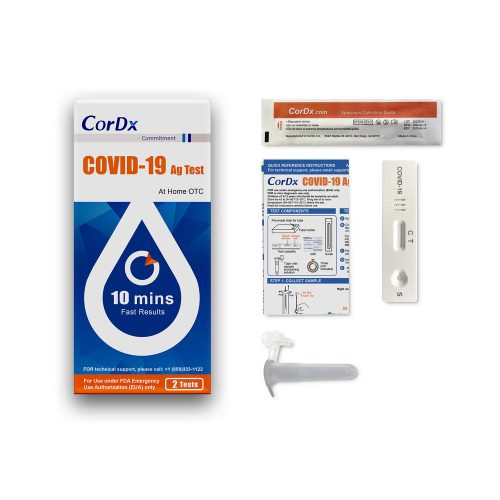
(Box) CorDx COVID-19 Antigen At-Home OTC, 8 Test/ Bx
SKU:
BB-DX03
(Box) Status™ Mono for whole blood Rapid Test Kit CLIA Waived, 25/Bx
SKU:
BB-MN01
(Box) Status iFOBT Rapid Test Kit CLIA Waived, 30/Bx
SKU:
BB-FB01
(Box) Status Strep A Plus Rapid Test Kit CLIA Waived, 50/Bx
SKU:
BB-SA03
(Box) Status iFOBT Mailers with Collection Tubes CLIA Waived, 25/Bx
SKU:
BB-FB02
(Box) Status H. pylori Rapid Test Kit CLIA Waived, 30/Bx
SKU:
BB-HP01
(Box) CorDx COVID-19 Antigen At-Home OTC, 2 Test/ Bx
SKU:
BB-DX01
The CorDx COVID-19 Ag Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal (nares) swab samples from individuals aged two years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 7 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests.
The CorDx COVID-19 Ag Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen, which is generally detectable in anterior nasal (nares) swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Individuals who test positive with the CorDx COVID-19 Ag Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.