Shop
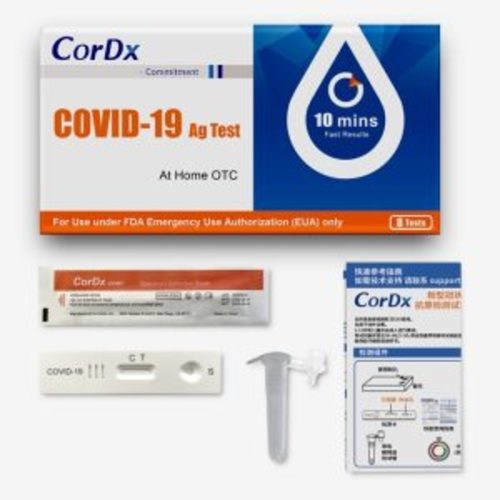
(Box) CorDx COVID-19 Antigen At-Home OTC, 8 Test/ Bx
SKU:
BB-DX03
(Each) Specimen Container 3oz/90 ml Sterile with Temp. strip & needle Individual pack
SKU:
BB-UC01
This collector is composed of Urine collection cup and vacuum urine collection tube, which is made of Medical grade Plastic material, mainly used for urine specimen collection. Good sealing performance prevents leakage effectively, convenient for specimen storage and transportation, also can avoid the contact between medical staff and specimen.
Safety and convenient to take the samples. Easy & Non pollution during the transportation.
The vacuum urine specimen collection cups are perfect for improving sample integrity with the leak-proof vacuum transfer design. They reduce the risk of urine contamination by patients or medical staff. Each 90 ml cup is individually packaged with a screw on top and are great for pneumatic transport with their watertight closing system.
(Each) Specimen Container 3oz/90 ml Sterile Individual pack (Urine Container)
SKU:
BB-UC02
Safer storage and transportation of samples, especially for biological samples has been critical, owing to the risk of potentially hazardous substances.
PP material, 90ml volume, Screw cap, molded graduation & sealed label
Straight-sided container format featuring top and bottom positive grip knurls for ease of opening and closing when wearing protective or latex gloves
Stackable, leak-resistant and graduated.
Sterile containers labeled with a security tab label and sterilized.
The cup that contains a leak-proof cap, label, integrity seal, and tamper evident tape to ensure sample integrity. Meets US Postal Service and private courier specifications for transporting urine specimens.
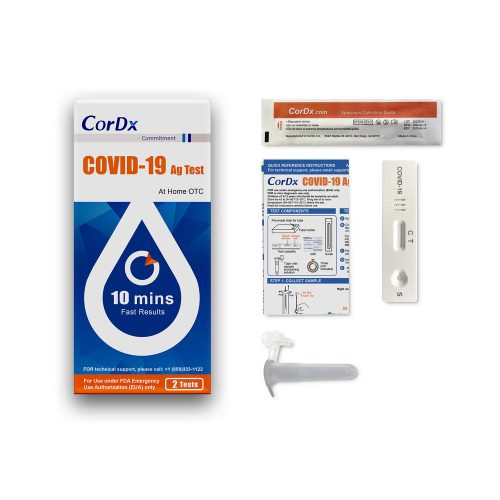
(Box) CorDx COVID-19 Antigen At-Home OTC, 2 Test/ Bx
SKU:
BB-DX01
The CorDx COVID-19 Ag Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal (nares) swab samples from individuals aged two years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 7 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests.
The CorDx COVID-19 Ag Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen, which is generally detectable in anterior nasal (nares) swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Individuals who test positive with the CorDx COVID-19 Ag Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.